Nano focus
An increasing number of workers are concerned by exposure to nanomaterials
According to various estimates, some 300,000 to 400,000 people are currently employed in the nanotechnologies industry in Europe. In France, over 5,000 workers in companies and 7,000 laboratory researchers might be exposed. Assessment of the effects of nanomaterials on worker health is a core concern of OSH specialists, both nationally and internationally, and it is one of the priority programmes of INRS.
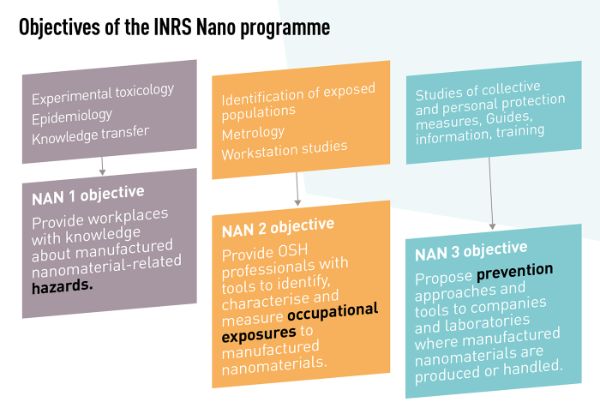
In 2008, INRS set up an action programme for nanomaterial-related risks. The primary aim of this work is to enhance knowledge (laboratory research and field studies) and transfer it to the workplace. The programme is designed to provide some answers to the challenges raised by nanomaterials.
The programme focuses on:
- the assessment of the dangers of nanomaterials to health via toxicological studies. Such studies are conducted primarily using inhalation tests to investigate properties which determine toxicity, the migration in cells or the body, and the genotoxic potential of nanomaterials;
- the characterisation of nanomaterials and occupational exposure to nanomaterials;
- the effectiveness of collective protective equipment and personal protective equipment;
- the management and perception of risks and communication on the subject.
The programme includes the acquisition of new equipment and the setting up of safe testing facilities. In 2013, INRS inaugurated its “Nano” research unit in order to bring together employees who are involved in research on nanomaterials in a dedicated location, facilitating exchanges and multi-disciplinary cooperation between the different teams. This new laboratory, approximately 600 square metres in size, offers:
- an area reserved for toxicology studies, nanoparticle generation and exposure of animals (rodents) by inhalation, meeting the regulatory requirements for animal testing;
- an ISO class 5 clean room initially dedicated to work on collective protective equipment;
- a zone including an area for the CAIMAN experimental facility and four areas equipped with safety cabinets and controlled environment chambers, for activities relating to nanoparticle characterisation and metrology, and respiratory protective devices.
In addition to this research, INRS is also conducting sociological studies, for example on workplace organisation and prevention measures in laboratories using manufactured nanomaterials.
Because prevention measures must be put in place even before more detailed answers about risks are known, INRS develops and recommends suitable prevention measures and finds the consensus necessary to assist companies. INRS publishes and distributes tools to raise awareness and provide information.
INRS has launched a process to establish appropriate threshold values for manufactured nanomaterials. A first article was published in March 2016 in the INRS journal Hygiène & Sécurité au Travail on nanoscale titanium dioxide, and research will continue in 2017 on carbon nanomaterials.
INRS also provides support and advice to companies and laboratories, particularly in regards to the characterisation of occupational exposure (by conducting on-site measurement campaigns) and the assessment and implementation of prevention solutions.
Lastly, INRS provides training for a wide and diverse audience.
The programme builds both on INRS's multidisciplinary teams, which bring together toxicologists, chemists, physicists, air flow experts, physicians and epidemiologists, and on external partnerships. Much of the work is carried out within the context of doctoral theses and cooperation, both nationally (national cooperation agreements with CEA, CNRS, ANSES, INERIS, etc.) and internationally (including European projects like NANoREG, NanoCEN, Nanogenotox and SmartNanoTox, OECD, PEROSH, etc.).
A foresight exercise on the development of nanomaterials by 20301 and their consequences on occupational safety and health in small enterprises in France took place in 2014. It was conducted in cooperation with French partners, the Swiss accident insurance fund (Suva) and the International Social Security Association (ISSA).1http://en.inrs.fr/inrs/strategic-plan/foresight-exercise.html
European Projects
-
European and international standardisation
INRS takes part in efforts towards the standardisation of nanomaterials by way of the ISO TC 229 and CEN TC 352 committees. Several of the many ongoing projects of these committees are closely related to action taken by INRS and more generally speaking are useful for prevention:
- General framework for the development of occupational exposure limits and bands for nano-objects and their aggregates and agglomerates (ISO 18637)
- Nanotechnologies – Nano-object aerosol generators for inhalation toxicity studies (ISO TR 19601)
- Nanotechnologies – Safety practices in occupational settings relevant to nanotechnologies (ISO TR 12885)
- Guide to the management of waste and the disposal of nanomaterials (00352014)
- Manufactured nanomaterials in the construction industry: guidelines for occupational risk management (00352023)
-
NANOGENOTOX
-
NANoREG
-
NANOCEN
-
SMARTNANOTOX

For download
- Proceedings of the foresight exercise on nanoparticles2
- Proceedings of the INRS international scientific conference Nanoparticle and nanomaterial-related risks3”, NANO 2011
INRS Brochures
- Nanomaterials “Definitions, toxicological risk, characterisation of occupational exposure and prevention measures”
- Nanomaterials “Definitions, toxicological risk, characterisation of occupational exposure and prevention measures”